A fresh approach to beat genital herpes
There is no vaccine for herpes. However, based on ground-breaking research from the University of Gothenburg in Sweden, Simplexia has developed a novel and unique vaccine approach to stop genital herpes virus by locking down the virus as it tries to spread between cells.
A novel strategy to develop a vaccine against HSV-2
Although several vaccine approaches have been tested in humans, none of them has led to a licensed vaccine. Simplexia offers a novel and unique approach to develop a vaccine against HSV-2 infection.
Vaccination – A Global Priority
Herpes virus infections are lifelong, incurable, often stigmatizing, and no existing interventions can effectively prevent them at the population-level. Vaccine development is a global WHO priority and fits several of the UN’s 17 Sustainable Development Goals.
Prevention is better than treatment
The therapeutic options available, such as oral antiviral drugs, only treat symptoms. Furthermore, they are only partially effective in reducing the effects of infection and the risk of transmission between partners. Oral antiviral drugs can be effective, reducing symptoms of HSV-2 by 70-80%. However, compliance can be a problem for suppressive treatment. Antiviral drugs have no effect or poor effect on recurrent HSV-2 meningitis, neonatal infections during childbirth, and do not reduce the risk of acquiring HIV, which is associated with HSV-2 infection. Continuous therapy with anti-herpesvirus medications is less than 50% effective in reducing transmission between partners. Even individuals without symptoms can transmit HSV-2. Approximately 20% of persons who test positive for HSV-2 do not show typical signs of infection. Untested, infected but symptom-free individuals may transmit the virus to partners without being aware of the risk. The development of one or more herpes simplex virus (HSV) vaccines has therefore been identified by the World Health Organization as an important goal for sexual and reproductive health (SRH) worldwide.
The Simplexia Approach
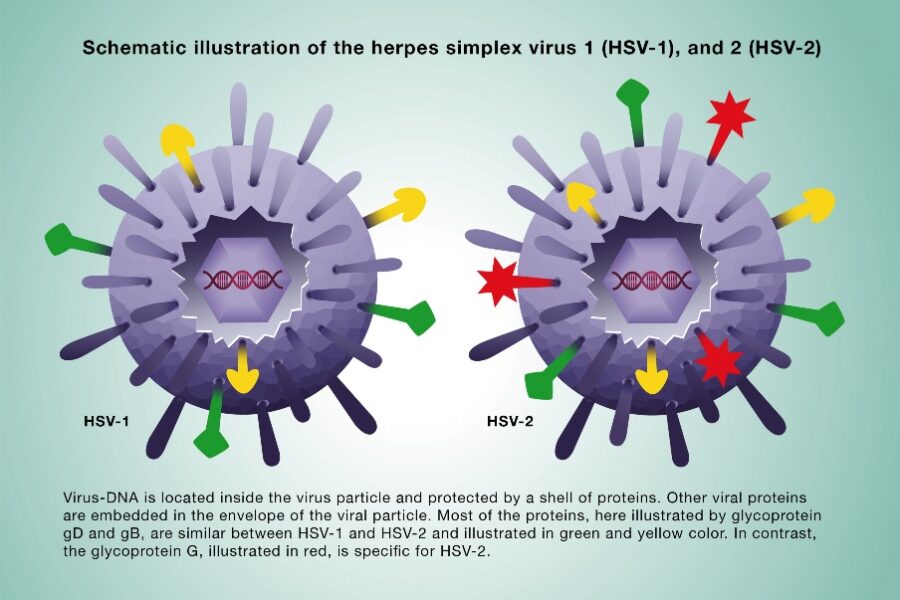
Cross-reactive immune responses from a prior HSV-1 infection do not provide protection against subsequent HSV-2 infection. Several human vaccine trials using cross-reactive antigens found on both HSV-1 and -2 virus have failed. Therefore, Simplexia’s vaccine is based on a type-specific protein of HSV-2, gG-2. Simplexia has developed a soluble version of the membrane-bound region of gG2 (mgG2) as an HSV-2 vaccine antigen.
Support for the Simplexia approach
In contrast to the successes achieved with the herpes zoster “shingles” vaccines, an effective vaccine for HSV has remained elusive. Although several vaccine approaches have been tested in humans, none of them has led to a licensed vaccine. In spite of promising results in pre-clinical studies in animal models, earlier candidates that have been tested in clinical trials have achieved only partial success.
Nevertheless, the herpes zoster vaccine has shown that protein subunit vaccines can be effective at stimulating an immune response against viral proteins and protecting against recurrent disease.
The scientific rationale
Simplexia has therefore chosen a novel approach to vaccine development for genital herpes based on research from the University of Gothenburg and from earlier pre-clinical and clinical data.
Type-specific antigen
Unlike other HSV-2 glycoproteins mgG-2 has low sequence homology with its HSV-1 counterpart and has been shown to only induce an HSV-2 type specific antibody response. In fact, the mgG-2 protein has been used for several years as a type-specific antigen for HSV-2 specific diagnosis. Since gG2 is unique for HSV-2, vaccination with Simplexia's vaccine is not affected by previous HSV-1 infection.
Role in release of virus from cells
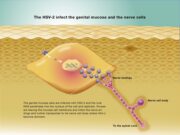
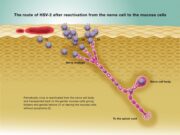
Virus studies using an mgG-2-negative HSV-2 mutant show that mgG-2 is involved in the extracellular release of infectious particles. Although glycoprotein gG2 is not essential for replication in cultured cells it is important for the exit of HSV-2 virus from cells and intact mgG-2 in clinical HSV-2 isolates is of importance for human infection, as mgG-2-negative HSV-2 isolates are rarely detected.
Stimulates proliferation of T-cells in infected patients
Proliferative helper T-cells responses are seen in HSV-2 infected patients after stimulation with gG2 protein, with greater responses seen in asymptomatic than symptomatic individuals.
Protects against disease in challenge model
Vaccination of mice with either mgG2 or Simplexia’s soluble version of mgG2 protects mice against genital HSV-2 infection in a laboratory challenge model.